Mon, Oct 31, 2022, 8:00AM Nuclear News James Conca
This article is from the American Nuclear Society Nuclear Newswire and written by James Conca.

The trouble with tritium is there is no trouble with tritium.
At any level outside the laboratory, either experimental or manufacturing, tritium is harmless. Every year, we routinely release millions of gallons of slightly tritiated water to the ocean, large lakes, and large rivers from almost every commercial nuclear reactor in the world, and have done so for decades, all in accordance with globally accepted nuclear safety standards. And no adverse effects on the environment or humans have ever been seen.
This is not surprising, since more tritium is created in Earth’s upper atmosphere than is created by commercial reactors. Natural (cosmogenic) tritium is continuously created in the upper atmosphere, mostly by the reaction
14N + n → 3H + 12C,
which forms 70 quadrillion becquerels, or approximately 2 million curies, of tritium (3H) every year (irsn.fr/EN/Research /publications-documentation/radionuclides -sheets/environment/Pages/Tritium-environment.aspx). Tritium then rains out into surface waters from which we end up drinking or fishing. Even more important, according to the Woods Hole Oceanographic Institute (waterencyclopedia.com/Po-Re /Radionuclides-in-the-Ocean.html), there are 74.1 sextillion becquerels of potassium-40, rubidium-87, and many other higher-energy emitters already in the world’s oceans.
But lately, Fukushima has brought the topic of tritium in the ocean to the public’s eye and, well, the horror and outrage have been quite a spectacle to witness. It’s as if the public just realized no one is recycling the glass bottles they so carefully wash and put in the recycle bin day after day.
A large commercial nuclear power reactor produces about 20,000 curies (~2 g) of tritium per year, most of which is incorporated into the nuclear fuel and coolant/control materials such as boron. Some enters the water, replaces an H in H2O to become HTO, and can leave the reactor in amounts dependent on the reactor design. Nuclear power plants routinely and safely release diluted concentrations of tritiated water. These authorized releases are closely monitored by the utilities and reported to the Nuclear Regulatory Commission, and the information is made available to the public on the NRC’s website. Each commercial nuclear power plant is required to submit two reports annually: one radioactive environmental operating report and another on radioactive effluent release.
Some years, there are no effluent releases from an operating nuclear power plant, but a typical release can range anywhere from thousands to millions of gallons per year, with a total tritium amount of only 10 curies or so.
So what is the effect of this discharge on humans, the body of water into which the tritiated water is discharged (usually the ocean), or the ecosystem?
First, tritium has the weakest radioactivity of any radionuclide, with an average beta emission of a measly 6 keV. It’s difficult for such a low-energy beta to get through the water, cell walls, and other materials in between the radionuclide and any DNA. The energy mostly gets dispersed within the electron clouds of other molecules like H2O through inelastic collisions and the bremsstrahlung effect, turning kinetic energy into electromagnetic nonionizing energy.
The half-life of tritium is short, with a physical half-life of just over 12 years and biological half-life of mere days (just under 10 days in humans; just over 2 days in fish). Both tritium and tritiated water thermodynamically prefer to remain in water. Tritium becomes more dilute as it moves up the food chain and therefore does not adversely affect the oysters, clams, or fish in the ocean—something the fishing industry needs to know. Organically bound tritium also thermodynamically prefers to be in water, is slightly slower than tritiated water to leave tissue, and has never been observed to maintain or increase in concentration as it moves up the food chain.
Tritium concentrations in seawater are about 0.7 Bq/l. For reference, our blood contains 250 Bq/l of radionuclides, 99% of which are about 100 times more energetic than tritium’s beta.
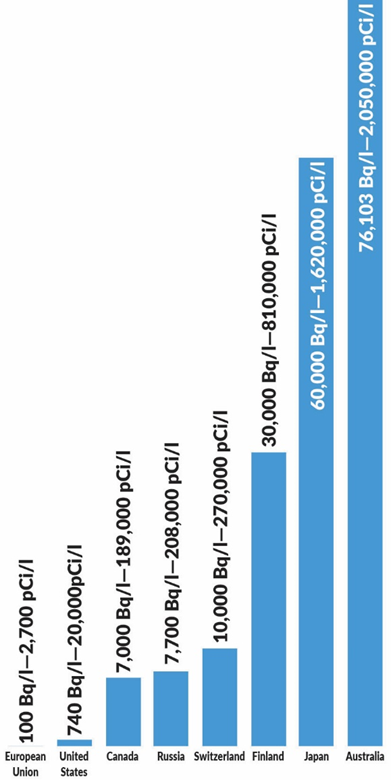
Tritium drinking water limits, by country
Tritium is so harmless that regulators do not know what maximum regulatory limits to set (see graph at right), though the World Health Organization has a recommended limit of 10,000 Bq/l (270,000 pCi/l). Discharging tritium from power plants has never resulted in the passing of any of these limits.
What do we know about the human health and environmental effects of tritium at the levels we see naturally occurring in the environment and after being discharged from nuclear power plants? Antone Brooks, a radiobiologist and environmental scientist who focused on the biological changes induced by low doses of ionizing radiation and authored the 2018 book Low-Dose Radiation, kindly provided the log-lines pictured below to illustrate the effects, both radiological and dose. Activity or dose increases by an order of magnitude going from left to right and from the bottom to the next line above.
As can be seen, activity levels in surface waters after average discharges are orders of magnitude below any regulatory levels for drinking water—not that anyone is going to drink seawater—and are many orders of magnitude below any observable health effects in live animals.
The same is true for the dose range plot. Even with poor mixing in the receiving body of water, say, only 1 percent effective mixing, the range of normal doses to humans after average discharges is well below any regulatory value, public or radiation worker, and is six orders of magnitude below any observable health effects in humans.
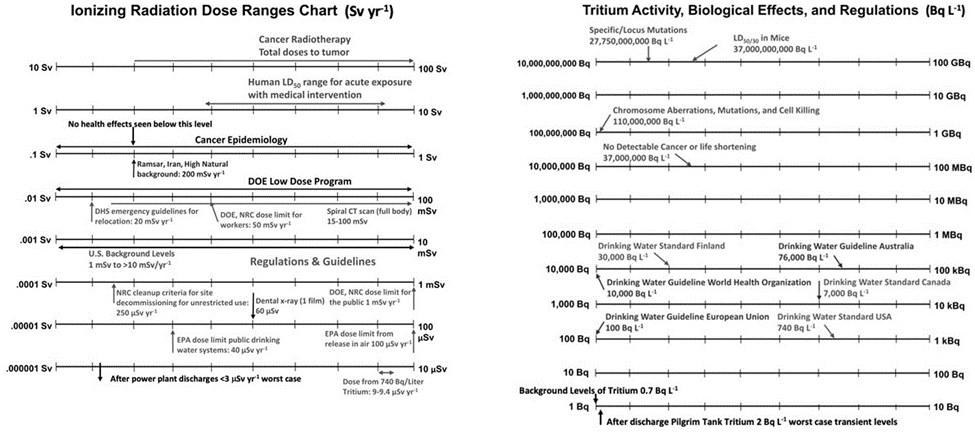
Graphics courtesy of Antone Brooks.
Of course, the public knows nothing of this and is skeptical even when told about the extremely low risk presented by tritium releases. I have experience in dealing with the public and have brought this to wider attention at venues such as Citizens Advisory Panel meetings for the decommissioning of power plants like San Onofre or Pilgrim. The distrust of government and even academic experts is so high as to be baffling.
But no adverse effects to human health or the environment has ever occurred from these releases, and we’ve been doing them for 60 years. I’m not sure what else there is to say in order to convince people.



