The Pacific Northwest National Laboratory (PNNL), located in Richland, Washington, and funded by the Department of Energy (DOE) has developed a new molten-salt battery design that would be a “safer and more scalable” energy storage system for the national grid.
It is built with low-cost metals, sodium and aluminum, and it has the potential to charge and discharge faster than other conventional high-temperature sodium batteries, operate at a lower temperature, and maintain an “excellent” energy storage capacity. During testing, after 345 charge/discharge cycles at high current, the battery’s acidic reaction mechanism retained 82.8% of its peak charge capacity.
PNNL researchers speculate that this battery could result in a practical specific energy density up to 100 Watt-hr/kg. (The energy that a battery delivers during its discharge process is called its specific energy density.) Although the specific energy density for lithium-ion batteries, used in commercial electronics and electric vehicles, is greater, around 170–250 Wh/kg. This new molten-salt battery design (sodium-aluminum battery) is inexpensive and easy to produce in the United States from much more abundant materials.
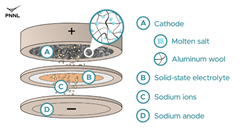
The battery’s cathode is composed of molten salt and aluminum wool (aluminum wool is a scrap product from aluminum manufacturing) and has a solid-state sodium-based electrolyte (supplied by Nexceris) that allows only sodium ions to travel from the negative (anode) to the positive (cathode) as it charges.
One of the PNNL’s design innovations was a change in the battery’s shape, from tubular to a flat scalable shape that can be stacked and expanded as technology develops, from its current coin-size to a larger grid-scale size. This flat design will also allow changes to the battery’s capacity, increasing its capacity by using a thicker cathode. In fact, the researchers modified the design to demonstrate a triple capacity cell that provided a sustained discharge of 28.2-hours under laboratory conditions.